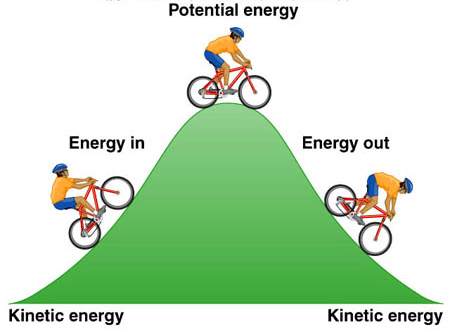
2. Potencial energy: energy stored in an object or material.

3.temperature: the average kinetic energy of the molecules in a material.
4.Heat: energy that flows between objects that have different temperatures.
5. Radiation: the transfer of energy by electromegnetic waves.
6.Conduction: the transfer of energy by direct contact of moleculas.

7. Convection: the transfer of energy by the flow of a liquid or gas.

Insulation:prevents heat from flowing in or out of a material.